Can microbes be used to treat or prevent disease?
Microbial biotherapeutics are revolutionizing medicine, and their use is expanding. Fecal microbiota transplants (FMTs) have been successful in improving health outcomes in some cases, but the mechanisms that modulate the efficacy of such treatments remain poorly understood [1]. Not all diseases may be treatable by transfaunation, as rewilding of whole microbiomes may introduce microorganisms that are not beneficial, but instead detrimental. One gap in our knowledge is what defines a healthy microbiome for most animal species [2]. Therefore, understanding the function of microorganisms is necessary to direct precision therapeutic restoration efforts. To fill this gap, I have begun sampling extensively across animals to create a biobank [3] that can be used to develop therapeutic treatments to improve host fitness through direct restoration of microbiota using fecal microbiota transplantation (FMT), live probiotics, or microbially-derived metabolite bio-therapeutics. Currently, I am working on three different lines of inquiry:
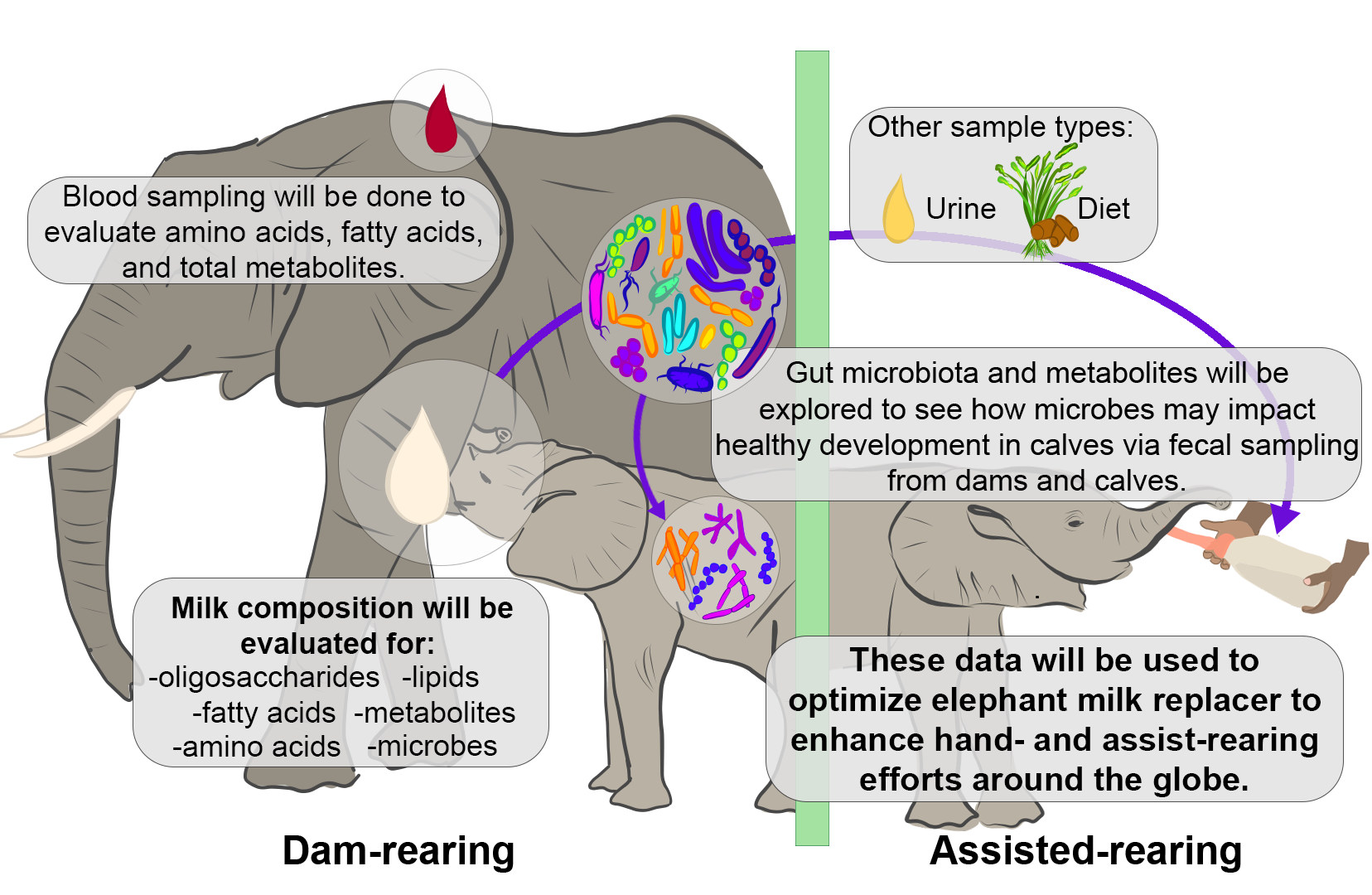
From milk to microbes: enhancing assist-rearing outcomes in African elephants through a multidisciplinary approach
For some species of hoofstock, hand- and assist-rearing endeavors are largely successful, but for others, such as African elephants (Loxodonta africana), success is limited—especially for young animals. Examining milk with longitudinal sampling will increase our knowledge of how nutrients in milk vary across lactation but also of how microbial colonization occurs, and together how changing nutrient composition in milk may also interact with gut microbiota to lead to different health outcomes. Together, we will integrate cutting-edge techniques to evaluate multiple sample types to better understand the role of milk components and microbes in the healthy development of calves of both African elephants and rhinos in managed care through intensive sample collection. Our goal is to use this information to aid in the development of an elephant milk replacer that will enhance hand- and assist-rearing in managed care around the globe.
Fecal microbiota transplants in rhinoceros
I am working with veterinarians to develop FMT protocols and evaluating their efficacy to treat chronic GI-disease in rhinoceros [4]. Despite the common use of such therapies in livestock, their mechanism of action and long-term effects are poorly understood. Using an age and sex-matched donor, we evaluated if FMTs shifted the microbiome, and if we observed any improvement in recipient condition. Using sequencing and untargeted metabolomics, we found that both the microbiota and metabolome shifted following FMTs, with the metabolome converging between donor and recipient after treatment, as well as the donor contributing a high proportion of bacterial taxa observed in the recipient. Despite these promising results, more work is needed to optimize timing of repeat treatments, hopefully leading to amelioration of chronic gastrointestinal imbalance.
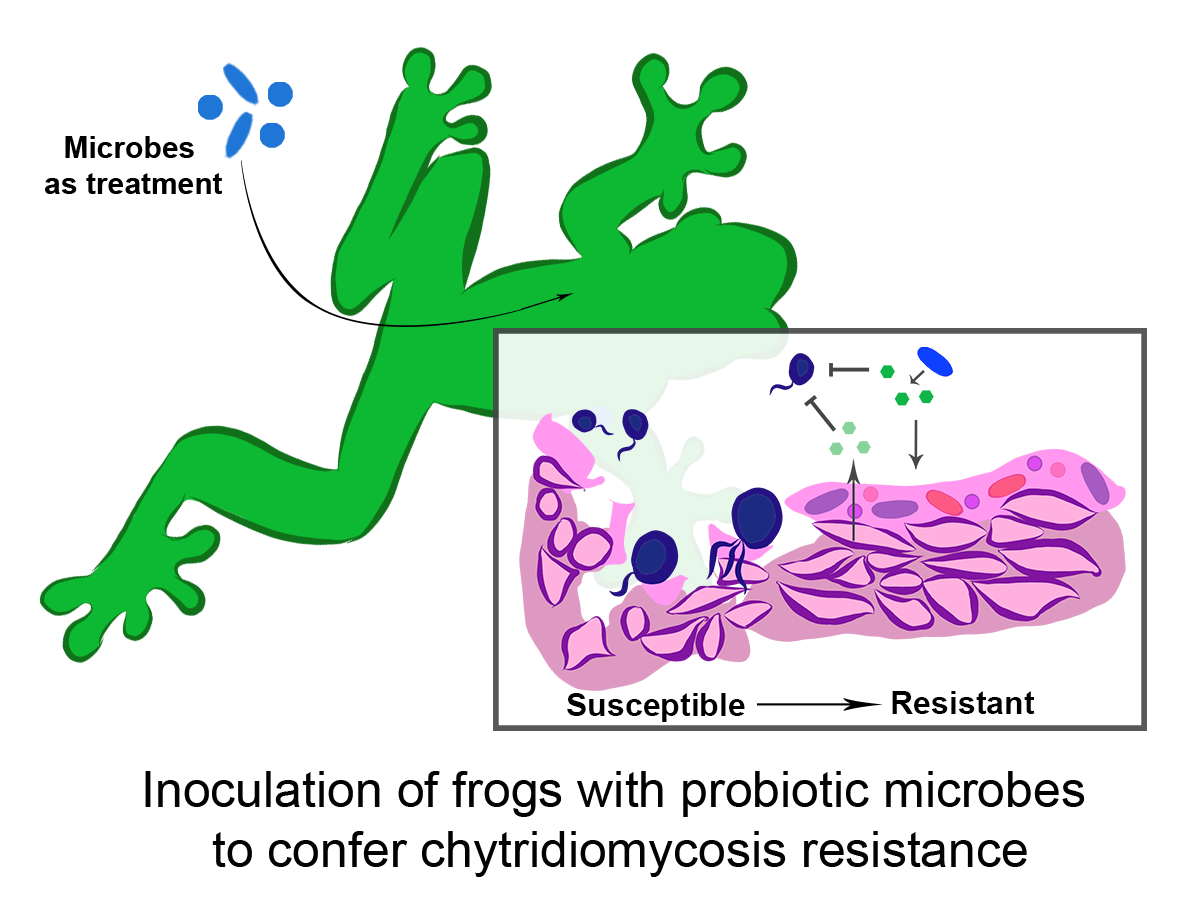
Bacterial augmentation to prevent Batrachochytrium dendrobatidis infection
I am also working with the endangered mountain yellow-legged frog (Rana muscosa) to evaluate the efficacy of probiotic skin treatments to confer disease resistance to Batrachochytrium dendrobatidis (Bd) after reintroduction. In order to do so, we have begun isolating bacteria from wild frogs, as well as Bd within wild populations [5]. We will evaluate which microbiota may be Bd-inhibitory, when is best to apply them, and what their potential protective mechanisms may be through multidisciplinary approaches spanning in vitro culture, metagenomics and metatranscriptomics, and metabolomics. Altogether, our goal is to develop population-specific treatments prior to reintroduction, like probiotic application or immune priming.
References
- Williams CL, Caraballo-Rodríguez AM, Allaband C, Zarrinpar A, Knight R, Gauglitz JM (2019). Wildlife-microbiome interactions and disease: exploring opportunities for disease mitigation across ecological scales, Drug Discovery Today: Disease Models, 28: 105-115.
- Williams CE, Hammer T, Williams CL (2023) Diversity alone does not reliably indicate the healthiness of an animal microbiome, in revision at ISME J.
- Calatayud, NE, Bragg J, Bruford M, van der Merwe, Brown A, Williams CL, Sole C, Hvilsom C, Steiner C, Russo IR (2023). IUCN Species Survival Commission Biobanking Guidelines, in preparation.
- Herrera MJ, Gauglitz JM, Kerr K, Marshall KL, Weldon K, Dorrestein PC, Singleton C, Williams CL (2023) Examining the potential of fecal microbiota transplants to treat gastrointestinal imbalance in greater one-horned rhinoceros, in preparation.
- Siddons S, Williams CL, Payne T, Hammond T, Shier D. Isolation and culturing of Batrachochytrium dendrobatidis for the conservation of the endangered mountain yellow-legged frog, Rana muscosa. Amphibian Disease meeting (virtual), November 2022.
